ALS & FTD
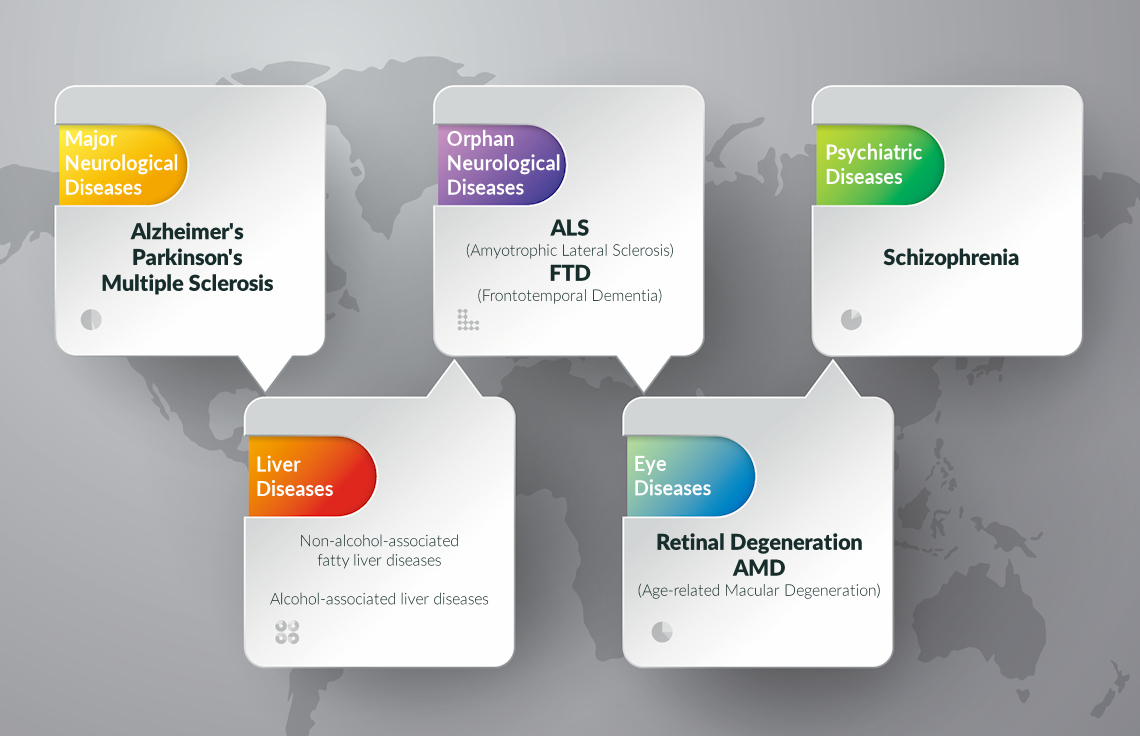
RAR modulators could contribute to protein metabolism and also modulate autophagic response. RAR-modulation correlates with axon outgrowth and nerve regeneration and maintenance.
RAR modulators could contribute to protein metabolism and also modulate autophagic response. RAR-modulation correlates with axon outgrowth and nerve regeneration and maintenance.
RAR-modulators are likely to be important for midbrain dopaminergic neurons since RARs and retinoic acid metabolising enzymes are highly expressed in these neurons and their target regions.
RAR deficiency is causative of AlzheimerŌĆÖs - vitamin A deficiency results in amyloid-beta deposition in cerebral blood vessels. We have shown many neuroprotective genes to be induced by RARs.